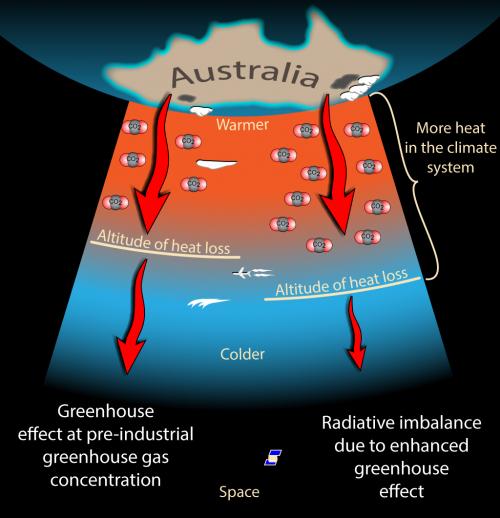
As I was writing about carbon dioxide levels rising in the previous post, I began asking myself what evidence we have to support that the rise is caused by fossil fuel burning by us – rather than from natural causes. That set me off down different paths – which I’ll explore with you here. I’m not an expert on any of these topics, but I know how to think about things in a scientific way – so here are my explorations.

First, I wondered about whether the carbon dating techniques would teach us about this. Carbon dating is a technique used to work out how old wooden objects are. It works like this: In the upper atmosphere, nitrogen atoms are hit by cosmic rays and are converted into carbon-14 (carbon atoms with 6 protons and 8 neutrons). Carbon-14 is radioactive and it decays, slowly, back to nitrogen (7 protons, 7 neutrons). If you have a large number of carbon-14 atoms, then after ~5730 years, half of them have decayed back to nitrogen (that’s what a half-life means). In the atmosphere, the cosmic rays keep making new carbon-14 atoms. A growing tree will take in carbon-14 as well as the other isotopes of carbon (carbon-12 and carbon-13) from the atmosphere while it is alive. Once it dies, there is no more carbon-14 coming in from the atmosphere but the carbon-14 that is in the wood continues to decay into nitrogen. So, if a boat or a chair was made from a tree, you can tell how old it is by seeing how much carbon-14 is left in it. Every ~5730 years the amount of carbon-14 halves.
Now, fossil fuels are fuels made from fossilised wood that grew hundreds of millions of years ago. So, there have been many, many half-lives that have passed, and there is no carbon-14 left. I wondered whether, as a result of us burning fossil fuels, the amount of carbon-14 in the air is noticeably lower than it “should be”?
I read quite a few online documents and scientific papers and discovered a couple of things – first that in the early 20th century there was a noticeable “ageing” of the atmosphere – it looked older than it should have done. But then we really messed up the readings by setting off lots and lots of atomic bombs.
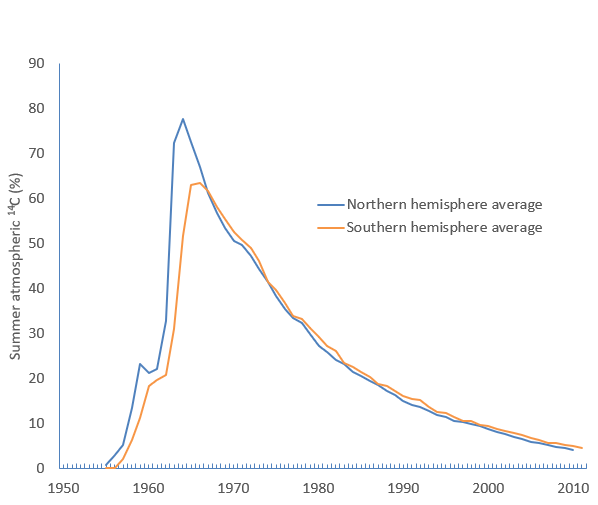
However, that’s now dropping and the scientific paper I found suggests that by 2050 brand new wood might look like it grew in 1050! I’m not completely sure whether that’s based on measurement or projection making the assumption that humans are emitting fossil carbon, but it does provide some evidence that you could test.
There’s also another carbon isotope, carbon-13. This is not radioactive, so doesn’t decay. From that you can tell something about the origin of the material. Photosynthesis affects the ratio of carbon-13 to carbon-12 as it prefers one to the other (I’m massively out of my depth with this chemistry and biology, so I’ll stop there – but apparently there are two types of photosynthesis). Whereas geological processes have no such bias. Therefore, if something was ever a plant, or ate a plant, the ratio is different than if it came from rocks. As a result you can distinguish fossil fuel carbon (from 100s of millions of years old trees that had photosynthesis) from volcano carbon. And the increase in carbon dioxide in the atmosphere shows it comes from plants – but ones that are old enough for carbon-14 to decay: in other words, fossil fuels.
We attempt to track carbon dioxide from volcanoes. There is no where near enough. Even if we’re a lot wrong in that, it’s not enough.
Also the oxygen levels are decreasing at the rate you’d expect if we were burning things. And we know carbon dioxide levels are increasing in the ocean, so it’s not ocean outgassing.
Other evidence that the increase in carbon dioxide comes from us comes from a simpler source – we know how much fossil fuel we’ve dug or pumped out of the ground. Because it has a monetary value, we actually track that very carefully. Basic chemistry tells us that carbon dioxide is a combustion product when we burn fossil fuels (we can also measure that in a laboratory easily). So we can calculate how much increase we’d expect. The increase in carbon dioxide in the atmosphere is quite a lot lower than what we’d expect from that simple calculation. That’s because the oceans and the trees have taken up a lot of our emissions. But not all. And measurements over them (e.g. by those satellites we talked about in the last lesson) show that they are now absorbing less (the oceans are “saturating” and simply can’t take any more and we’re cutting down, rather than planting, forests). The global climate budget tries to track and measure all this.
(I promise a later blog called “But dinosaurs didn’t drive SUVs” to discuss why carbon dioxide levels were much higher in their days without us).